From Wikipedia, the free encyclopedia
Not to be confused with Carbon fiber.
| Part of a series of articles on |
| Nanomaterials |
|---|
| Fullerenes |
| Nanoparticles |
| Nanotechnology portal' |
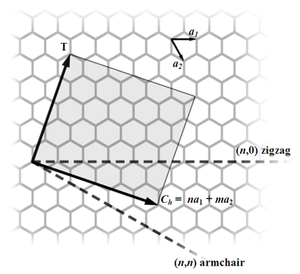
Nanotubes are members of the fullerene structural family. Their name is derived from their long, hollow structure with the walls formed by one-atom-thick sheets of carbon, called graphene. These sheets are rolled at specific and discrete ("chiral") angles, and the combination of the rolling angle and radius decides the nanotube properties; for example, whether the individual nanotube shell is a metal or semiconductor. Nanotubes are categorized as single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs). Individual nanotubes naturally align themselves into "ropes" held together by van der Waals forces, more specifically, pi-stacking.
Applied quantum chemistry, specifically, orbital hybridization best describes chemical bonding in nanotubes. The chemical bonding of nanotubes is composed entirely of sp2 bonds, similar to those of graphite. These bonds, which are stronger than the sp3 bonds found in alkanes and diamond, provide nanotubes with their unique strength.
Contents
- 1 Types of carbon nanotubes and related structures
- 2 Properties
- 3 Synthesis
- 4 Current applications
- 5 Potential applications
- 6 Discovery
- 7 See also
- 8 References
- 9 External links
Terminology
There is no consensus on some terms describing carbon nanotubes in scientific literature: both "-wall" and "-walled" are being used in combination with "single", "double", "triple" or "multi", and the letter C is often omitted in the abbreviation; for example, multi-walled carbon nanotube (MWNT).Single-walled

A scanning tunneling microscopy image of single-walled carbon nanotube

A transmission electron microscopy image of a single-walled carbon nanotube
SWNTs are an important variety of carbon nanotube because most of their properties change significantly with the (n,m) values, and this dependence is non-monotonic (see Kataura plot). In particular, their band gap can vary from zero to about 2 eV and their electrical conductivity can show metallic or semiconducting behavior. Single-walled nanotubes are likely candidates for miniaturizing electronics. The most basic building block of these systems is the electric wire, and SWNTs with diameters of an order of a nanometer can be excellent conductors.[3][4] One useful application of SWNTs is in the development of the first intermolecular field-effect transistors (FET). The first intermolecular logic gate using SWCNT FETs was made in 2001.[5] A logic gate requires both a p-FET and an n-FET. Because SWNTs are p-FETs when exposed to oxygen and n-FETs otherwise, it is possible to protect half of an SWNT from oxygen exposure, while exposing the other half to oxygen. This results in a single SWNT that acts as a NOT logic gate with both p and n-type FETs within the same molecule.
Single-walled nanotubes are dropping precipitously in price, from around $1500 per gram as of 2000 to retail prices of around $50 per gram of as-produced 40–60% by weight SWNTs as of March 2010.[citation needed]
Multi-walled

A scanning electron microscopy image of carbon nanotubes bundles
Double-walled carbon nanotubes (DWNT) form a special class of nanotubes because their morphology and properties are similar to those of SWNT but their resistance to chemicals is significantly improved. This is especially important when functionalization is required (this means grafting of chemical functions at the surface of the nanotubes) to add new properties to the CNT. In the case of SWNT, covalent functionalization will break some C=C double bonds, leaving "holes" in the structure on the nanotube and, thus, modifying both its mechanical and electrical properties. In the case of DWNT, only the outer wall is modified. DWNT synthesis on the gram-scale was first proposed in 2003[6] by the CCVD technique, from the selective reduction of oxide solutions in methane and hydrogen.
The telescopic motion ability of inner shells[7] and their unique mechanical properties[8] will permit the use of multi-walled nanotubes as main movable arms in coming nanomechanical devices. Retraction force that occurs to telescopic motion caused by the Lennard-Jones interaction between shells and its value is about 1.5 nN.[9]
Torus
A stable nanobud structure
Nanobud
Carbon nanobuds are a newly created material combining two previously discovered allotropes of carbon: carbon nanotubes and fullerenes. In this new material, fullerene-like "buds" are covalently bonded to the outer sidewalls of the underlying carbon nanotube. This hybrid material has useful properties of both fullerenes and carbon nanotubes. In particular, they have been found to be exceptionally good field emitters. In composite materials, the attached fullerene molecules may function as molecular anchors preventing slipping of the nanotubes, thus improving the composite’s mechanical properties.Graphenated carbon nanotubes (g-CNTs)
Graphenated CNTs are a relatively new hybrid that combines graphitic foliates grown along the sidewalls of multiwalled or bamboo style CNTs. Yu et al.[12] reported on "chemically bonded graphene leaves" growing along the sidewalls of CNTs. Stoner et al.[13] described these structures as "graphenated CNTs" and reported in their use for enhanced supercapacitor performance. Hsu et al. further reported on similar structures formed on carbon fiber paper, also for use in supercapacitor applications.[14] The foliate density can vary as a function of deposition conditions (e.g. temperature and time) with their structure ranging from few layers of graphene (< 10) to thicker, more graphite-like.[15]The fundamental advantage of an integrated graphene-CNT structure is the high surface area three-dimensional framework of the CNTs coupled with the high edge density of graphene. Graphene edges provide significantly higher charge density and reactivity than the basal plane, but they are difficult to arrange in a three-dimensional, high volume-density geometry. CNTs are readily aligned in a high density geometry (i.e., a vertically aligned forest)[16] but lack high charge density surfaces—the sidewalls of the CNTs are similar to the basal plane of graphene and exhibit low charge density except where edge defects exist. Depositing a high density of graphene foliates along the length of aligned CNTs can significantly increase the total charge capacity per unit of nominal area as compared to other carbon nanostructures.[17]
Nitrogen Doped Carbon Nanotubes
Nitrogen doped carbon nanotubes (N-CNT's), can be produced through 5 main methods, Chemical Vapor Deposition,[18][19] high-temperature and high-pressure reactions, gas-solid reaction of amorphous carbon with NH3 at high temperature,[20] solid reaction,[21] and solvothermal synthesis.[22]N-CNTs can also be prepared by a CVD method of pyrolysizing melamine under Ar at elevated temperatures of 800oC - 980oC. However synthesis via CVD and melamine results in the formation of bamboo structured CNTs. XPS spectra of grown N-CNT's reveals nitrogen in five main components, pyridinic nitrogen, pyrrolic nitrogen, quaternary nitrogen, and nitrogen oxides. Furthermore synthesis temperature affects the type of nitrogen configuration.[23]
Nitrogen doping plays a pivotal role in Lithium storage. N-doping provides defects in the walls of CNT's allowing for Li ions to diffuse into interwall space. It also increases capacity by providing more favorable bind of N-doped sites. N-CNT's are also much more reactive to metal oxide nanoparticle deposition which can further enhance storage capacity, especially in anode materials for Li-ion batteries.[24] However Boron doped nanotubes have been shown to make batteries with triple capacity.[25]
Peapod
A Carbon peapod[26][27] is a novel hybrid carbon material which traps fullerene inside a carbon nanotube. It can possess interesting magnetic properties with heating and irradiating. It can also be applied as an oscillator during theoretical investigations and predictions.[28][29]Cup-stacked carbon nanotubes
Cup-stacked carbon nanotubes (CSCNTs) differ from other quasi-1D carbon structures, which normally behave as quasi-metallic conductors of electrons. CSCNTs exhibit semiconducting behaviors due to the stacking microstructure of graphene layers.[30]Extreme carbon nanotubes
The observation of the longest carbon nanotubes (18.5 cm long) was reported in 2009. These nanotubes were grown on Si substrates using an improved chemical vapor deposition (CVD) method and represent electrically uniform arrays of single-walled carbon nanotubes.[1]The shortest carbon nanotube is the organic compound cycloparaphenylene, which was synthesized in early 2009.[31][32][33]
The thinnest carbon nanotube is armchair (2,2) CNT with a diameter of 3 Å. This nanotube was grown inside a multi-walled carbon nanotube. Assigning of carbon nanotube type was done by combination of high-resolution transmission electron microscopy (HRTEM), Raman spectroscopy and density functional theory (DFT) calculations.[34]
The thinnest freestanding single-walled carbon nanotube is about 4.3 Å in diameter. Researchers suggested that it can be either (5,1) or (4,2) SWCNT, but exact type of carbon nanotube remains questionable.[35] (3,3), (4,3) and (5,1) carbon nanotubes (all about 4 Å in diameter) were unambiguously identified using more precise aberration-corrected high-resolution transmission electron microscopy. However, they were found inside of double-walled carbon nanotubes.[36]
Properties
Strength
Carbon nanotubes are the strongest and stiffest materials yet discovered in terms of tensile strength and elastic modulus respectively. This strength results from the covalent sp2 bonds formed between the individual carbon atoms. In 2000, a multi-walled carbon nanotube was tested to have a tensile strength of 63 gigapascals (GPa).[37] (For illustration, this translates into the ability to endure tension of a weight equivalent to 6422 kg (14,158 lbs) on a cable with cross-section of 1 mm2.) Further studies, such as one conducted in 2008, revealed that individual CNT shells have strengths of up to ~100 GPa, which is in agreement with quantum/atomistic models.[38] Since carbon nanotubes have a low density for a solid of 1.3 to 1.4 g/cm3,[39] its specific strength of up to 48,000 kN·m·kg−1 is the best of known materials, compared to high-carbon steel's 154 kN·m·kg−1.Under excessive tensile strain, the tubes will undergo plastic deformation, which means the deformation is permanent. This deformation begins at strains of approximately 5% and can increase the maximum strain the tubes undergo before fracture by releasing strain energy.
Although the strength of individual CNT shells is extremely high, weak shear interactions between adjacent shells and tubes leads to significant reductions in the effective strength of multi-walled carbon nanotubes and carbon nanotube bundles down to only a few GPa’s.[40] This limitation has been recently addressed by applying high-energy electron irradiation, which crosslinks inner shells and tubes, and effectively increases the strength of these materials to ~60 GPa for multi-walled carbon nanotubes[38] and ~17 GPa for double-walled carbon nanotube bundles.[40]
CNTs are not nearly as strong under compression. Because of their hollow structure and high aspect ratio, they tend to undergo buckling when placed under compressive, torsional, or bending stress.[41]
| Material | Young's modulus (TPa) | Tensile strength (GPa) | Elongation at break (%) |
|---|---|---|---|
| SWNTE | ~1 (from 1 to 5) | 13–53 | 16 |
| Armchair SWNTT | 0.94 | 126.2 | 23.1 |
| Zigzag SWNTT | 0.94 | 94.5 | 15.6–17.5 |
| Chiral SWNT | 0.92 | ||
| MWNTE | 0.2[37]–0.8[46]–0.95[37] | 11[37]–63[37]–150[46] | |
| Stainless steelE | 0.186[47]–0.214[48] | 0.38[47]–1.55[48] | 15–50 |
| Kevlar–29&149E | 0.06–0.18[49] | 3.6–3.8[49] | ~2 |
The above discussion referred to axial properties of the nanotube, whereas simple geometrical considerations suggest that carbon nanotubes should be much softer in the radial direction than along the tube axis. Indeed, TEM observation of radial elasticity suggested that even the van der Waals forces can deform two adjacent nanotubes.[50] Nanoindentation experiments, performed by several groups on multiwalled carbon nanotubes[51][52] and tapping/contact mode atomic force microscope measurement performed on single-walled carbon nanotube,[53] indicated Young's modulus of the order of several GPa confirming that CNTs are indeed rather soft in the radial direction.
Hardness
Standard single-walled carbon nanotubes can withstand a pressure up to 25 GPa without deformation. They then undergo a transformation to superhard phase nanotubes. Maximum pressures measured using current experimental techniques are around 55 GPa. However, these new superhard phase nanotubes collapse at an even higher, albeit unknown, pressure.The bulk modulus of superhard phase nanotubes is 462 to 546 GPa, even higher than that of diamond (420 GPa for single diamond crystal).[54]
Kinetic properties
Multi-walled nanotubes are multiple concentric nanotubes precisely nested within one another. These exhibit a striking telescoping property whereby an inner nanotube core may slide, almost without friction, within its outer nanotube shell, thus creating an atomically perfect linear or rotational bearing. This is one of the first true examples of molecular nanotechnology, the precise positioning of atoms to create useful machines. Already, this property has been utilized to create the world's smallest rotational motor.[55] Future applications such as a gigahertz mechanical oscillator are also envisaged.Electrical properties
However, this rule has exceptions, because curvature effects in small diameter carbon nanotubes can strongly influence electrical properties. Thus, a (5,0) SWCNT that should be semiconducting in fact is metallic according to the calculations. Likewise, vice versa—zigzag and chiral SWCNTs with small diameters that should be metallic have finite gap (armchair nanotubes remain metallic).[56] In theory, metallic nanotubes can carry an electric current density of 4 × 109 A/cm2, which is more than 1,000 times greater than those of metals such as copper,[57] where for copper interconnects current densities are limited by electromigration.
Because of their nanoscale cross-section, electrons propagate only along the tube's axis and electron transport involves quantum effects. As a result, carbon nanotubes are frequently referred to as one-dimensional conductors. The maximum electrical conductance of a single-walled carbon nanotube is 2G0, where G0 = 2e2/h is the conductance of a single ballistic quantum channel.[58]
There have been reports of intrinsic superconductivity in carbon nanotubes.[59][60][61] Many other experiments, however, found no evidence of superconductivity, and the validity of these claims of intrinsic superconductivity remains a subject of debate.[62]
Optical properties
Main article: Optical properties of carbon nanotubes
EM Wave absorption
One of the more recently researched properties of multi-walled carbon nanotubes (MWNTs) is their wave absorption characteristics, specifically microwave absorption. Interest in this research is due to the current military push for radar absorbing materials (RAM) to better the stealth characteristics of aircraft and other military vehicles. There has been some research on filling MWNTs with metals, such as Fe, Ni, Co, etc., to increase the absorption effectiveness of MWNTs in the microwave regime. Thus far, this research has shown improvements in both maximum absorption and bandwidth of adequate absorption. The reason the absorptive properties changed when filled is that the complex permeability (μr) and complex permitivity (εr), shown in the equations below, have been shown to vary depending on how the MWNTs are called and what medium they are suspended in. The direct relationship between μr, εr, and the other system parameters that affect the absorption sample thickness, d, and frequency, f, is shown in the equations below, where Zin is the normalized input impedance. As shown in the equation below, these characteristics vary by frequency. Because of this, it is convenient to set a baseline reflection loss (R.L.) that is deemed effective and determine the bandwidth within a given frequency that produces the desired reflection loss. A common R.L. to use for this bandwidth determination is −10 dB, which corresponds to a loss of over 90% of the incoming wave. This bandwidth is usually maximized at the same time as the absorption is. This is done by satisfying the impedance matching condition, getting Zin = 1. In the work done at Beijing Jiaotong University it was found that Fe filled MWNTs exhibited a maximum reflection loss of −22.73 dB and had a bandwidth of 4.22 GHz for a reflection loss of −10 dB.![\begin{align}
& R.L.(dB)=20{{\log }_{10}}\left[ \frac{{{Z}_{in}}-1}{{{Z}_{in}}+1} \right] \\
& {{Z}_{in}}=\sqrt{\frac{{{\mu }_{r}}}{{{\varepsilon }_{r}}}}\tanh \left[ j\left( \frac{2\pi fd}{c} \right)\sqrt{{{\mu }_{r}}{{\varepsilon }_{r}}} \right] \\
\end{align}](http://upload.wikimedia.org/math/1/d/c/1dcbd91d985a22472e877cb60cffeceb.png)
Thermal properties
Main article: Thermal properties of nanostructures
All nanotubes are expected to be very good thermal conductors along the tube, exhibiting a property known as "ballistic conduction",
but good insulators laterally to the tube axis. Measurements show that a
SWNT has a room-temperature thermal conductivity along its axis of
about 3500 W·m−1·K−1;[63] compare this to copper, a metal well known for its good thermal conductivity, which transmits 385 W·m−1·K−1. A SWNT has a room-temperature thermal conductivity across its axis (in the radial direction) of about 1.52 W·m−1·K−1,[64]
which is about as thermally conductive as soil. The temperature
stability of carbon nanotubes is estimated to be up to 2800 °C in vacuum and about 750 °C in air.[65]










No comments:
Post a Comment