Scientific Method / Science & Exploration
http://arstechnica.com/
New formulation of perovskites can be tuned to wavelengths that work with silicon.
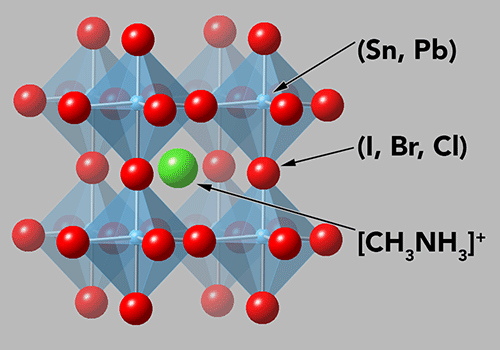
A typical perovskite, similar in structure to the one being tested here.
That's one of the reasons researchers have been trying to develop perovskites. Not only are these made from chemicals that are cheap and easy to manufacture, there are indications that they can be tuned to absorb some wavelengths while allowing others to pass through to an underlying silicon photovoltaic. The big problem: they tend to decompose when exposed to intense light.
Now, an Oxford-Berlin collaboration is reporting they may have solved the decomposition problem and, in the process, accidentally made a material where they could tune the absorbance across a wide range of wavelengths. With some additional improvements, they suggest a combined silicon-perovskite cell could reach 30 percent efficiencies—up from the neighborhood of 22 for silicon alone.
Perovskites aren't actually a single material; instead, they're a variety of chemicals that all form a similar crystal structure. For photovoltaics, they're often a mix of lead, bromine or iodine, and a small nitrogen-containing organic molecule. None of these is very expensive, and it's relatively easy to form a layer of perovskite materials using bulk techniques. The best of these materials has photovoltaic efficiencies in the teens. That's lower than silicon, but expectations are that it can be brought up even higher.
But the new paper doesn't focus on stand-alone perovskites; its authors are interested in combining them with silicon photovoltaics. For that to work, the bandgap of the perovskite and that of the silicon have to be distinct so that they absorb photons at different wavelengths. Since a variety of chemicals can form a perovskite-like structure, then simply finding the right combination should allow us to tune the perovskite so that it works with silicon.
The researchers began with a pretty standard perovskite starting material: a mixture of lead, iodine, bromine, and the organic molecule HC(NH2)2. This material had been tested before but found to suffer a problem typical of other perovskites—they break down when exposed to light and/or high temperatures. In this case, the material is unstable at temperatures above 85 degrees Celsius, which causes internal rearrangements to the crystal structure that eliminate its perovskite-ness.
The authors began by substituting some cesium for a portion of organic molecule, figuring it could push the temperature limit a bit higher. Instead, nearly every combination they tested was largely stable, although they only tested exposure to light for about an hour (something that needs significant follow-up). The team was also able to vary the bromine and iodine composition at the same time.
By tweaking these two chemical ratios (Cs to HC(NH2)2 and I to Br), they were able to fine tune the band gap of the material. To work well with silicon, they were targeting a bandgap of 1.75 electronVolts; they were able to make perovskites with a 1.74eV bandgap. "We have total flexibility and predictability in tuning the composition and its impact on the band gap," the researchers conclude.
On their own, these films exhibited a photovoltaic efficiency of nearly 15 percent—pretty good for a perovskite. When placed in front of silicon PV hardware, the silicon's efficiency dropped from 19 percent to a bit over seven percent. That's a pretty big drop, and it suggests that a two-layer design of this sort would only reach about a 20 percent efficiency, which is not much better than the silicon on its own.
However, the authors suggest that it would be relatively easy to reduce the opacity of the perovskite and design its cells to more actively manage the light passing through them. Different silicon cells could potentially boost the efficiency even more. The end result, they suggest, is that a 30-percent efficient two-layer cell could be attainable.
Right now, we already have cells with these sorts of efficiencies; they're just too expensive for widespread deployment. But if a perovskite layer could be added at a relatively low cost, it could shift the economics of installing photovoltaic hardware. The panels would be a bit more expensive, but they would produce a lot more electricity for the same installment costs.
Science, 2015. DOI: 10.1126/science.aad5845 (About DOIs).
No comments:
Post a Comment