Scientific Method / Science & Exploration
http://arstechnica.com/
It's taken 80 years, but high pressure may be breaking down hydrogen molecules.
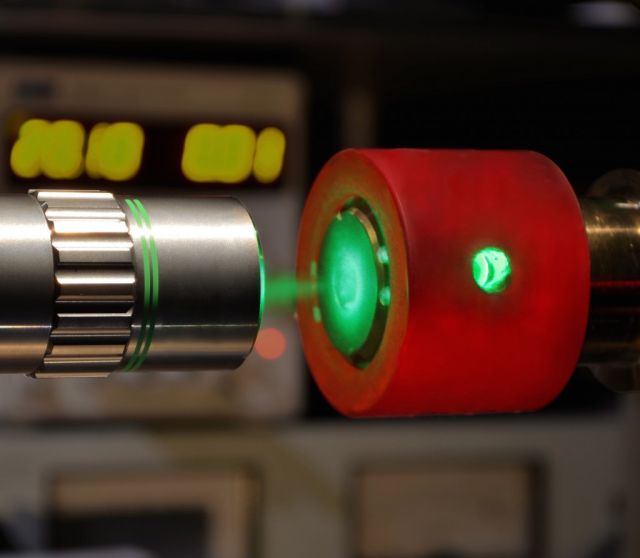
Raman spectroscopy and a diamond anvil were used to identify
and characterize a new phase of hydrogen. Philip Dalladay-Simpson
and Eugene Gregoryanz
Close to the cores of gas giants, elements are squeezed by unimaginable pressures, capable of rearranging electron orbitals and playing havoc with the chemical bonding we see on Earth. Here, theorists have predicted, the electrons of hydrogen could be set free, converting the gas into a solid or liquid metal. But while researchers first predicted the existence of metallic hydrogen 80 years ago, the element has stubbornly refused to appear, even after we've raised pressures well above where it was expected to appear.
Now, three Edinburgh-based researchers report placing hydrogen under the highest pressures yet achieved. Scans of the resulting material suggest that the chemical bonds that normally link hydrogen into a molecule are starting to break down, heralding the possible appearance of a metallic form.
Like other high-pressure work, the new experiments rely on what are called diamond anvils, which use two diamonds to crush any material placed between them. This hardware can apply absurd pressure to samples. For the experiments reported here, the material being crushed reached pressures of over 380 GigaPascals—with each GigaPascal being roughly equivalent to 10,000 atmospheres of pressure. These are the highest pressures achieved with hydrogen and among the highest pressures ever reported.
(The researchers tested both hydrogen and its heavier isotope, deuterium, as well as a mixture of the two isotopes.)
Normally, hydrogen comes in a molecular form, with two atoms sharing their electrons. This bond keeps the electrons from circulating freely and determines many of the molecule's properties. These properties include things like the wavelengths of light it absorbs as the electrons change their energy levels or the bond between them stretches. Making a metal involves dissolving that bond and therefore changing the properties.
Under visible light, hydrogen is normally transparent—it doesn't absorb any of these wavelengths. But that changed under extreme pressure, which the authors took as a possible sign that the bonding was breaking down: "The possible appearance of conducting electrons due to dissociation could explain the very dark appearance of the sample as seen in transmitted and reflected light in the visible region."
They also used Raman spectroscopy to look at the behavior of the bond, as this can provide information on its vibrations. At these pressures, two modes of vibration present in hydrogen molecules start to disappear, and the intensities of the remaining vibrational modes begin to decrease rapidly.
Since some signs of a bond are still present, the authors posit that this represents a new phase of hydrogen accompanying the gas and more readily achieved liquid forms. Because of some other exotic phases discovered in the search for hydrogen metal, this one is named phase V. The authors don't think that it represents a fully metallic form, since there are some signs that the bonds between hydrogen atoms are still present. But they're clearly not as robust as they were, so the authors speculate that it represents "the onset of the predicted non-molecular and metallic state of hydrogen."
The researchers also change the temperature of the sample and show that the boundaries between this new phase and phase IV are purely pressure-based—it doesn't seem to matter what the temperature is; the transition takes place at about 320 GigaPascals. This was taken as evidence that raising the temperature might create a form of hydrogen that's essentially a liquid metal.
The very weak signs of a chemical bond that remain suggest that it may only take a bit more pressure to get fully metallic hydrogen. But given the history of the field, I'd imagine many people will believe it when they see it.
Nature, 2015. DOI: 10.1038/nature16164 (About DOIs).
No comments:
Post a Comment