From Wikipedia, the free encyclopedia
| Sarin[1] | |
|---|---|
 |
|
 |
|
| Identifiers | |
| CAS number | 107-44-8 |
| PubChem | 7871 |
| ChemSpider | 7583 |
| UNII | B4XG72QGFM |
| ChEMBL | CHEMBL509554 |
| Jmol-3D images | Image 1 |
| Properties | |
| Molecular formula | C4H10FO2P |
| Molar mass | 140.09 g mol−1 |
| Appearance | Clear colorless liquid |
| Odor | Odorless in pure form |
| Density | 1.0887 g/cm³ (25 °C) 1.102 g/cm³ (20 °C) |
| Melting point |
-56 °C, 217 K, -69 °F |
| Boiling point |
158 °C, 431 K, 316 °F |
| Solubility in water | Miscible |
| Hazards | |
| MSDS | Lethal Nerve Agent Sarin (GB) |
| EU classification | Extremely Toxic (T+)[3] |
| Main hazards | It is a lethal cholinergic agent. |
| NFPA 704 | |
| LD50 | 70 mg-min/m3 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Sarin can be lethal even at very low concentrations, with death following within one minute after direct ingestion due to suffocation from lung muscle paralysis, unless some antidotes, typically atropine or Biperiden and pralidoxime, are quickly administered to a person.[4] People who absorb a non-lethal dose, but do not receive immediate medical treatment, may suffer permanent neurological damage.
Contents
Production and structure
Sarin is a chiral molecule because it has four chemically different substituents attached to the tetrahedral phosphorus center.[6] The SP form (the (–) optical isomer) is the more active enantiomer due to its greater binding affinity to acetylcholinesterase.[7][8] The P-F bond is easily broken by nucleophilic agents, such as water and hydroxide. At high pH, sarin decomposes rapidly to nontoxic phosphonic acid derivatives. It is usually manufactured and weaponized as a racemic mixture—an equal mixture of both enantiomeric forms—by the alcoholysis reaction of methylphosphonyl difluoride with isopropyl alcohol: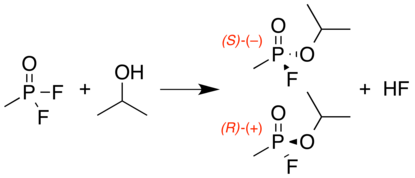
Isopropylamine is also included in the reaction to neutralize the hydrogen fluoride byproduct. As a binary chemical weapon, it can be generated in situ by this same reaction.
A by-product of sarin production is diisopropyl methylphosphonate (DIMP), which degrades into isopropyl methylphosphonic acid (IMPA).[9]
Biological effects
Like other nerve agents, sarin attacks the nervous system by stopping nerve endings in muscles from switching off. Death will usually occur as a result of asphyxia due to the inability to control the muscles involved in breathing function.Specifically, sarin is a potent inhibitor of acetylcholinesterase,[10] an enzyme that degrades the neurotransmitter acetylcholine after it is released into the synaptic cleft. In vertebrates, acetylcholine is the neurotransmitter used at the neuromuscular junction, where signals are transmitted between neurons from the central nervous systems to muscle fibres. Normally, acetylcholine is released from the neuron to stimulate the muscle, after which it is degraded by acetylcholinesterase, allowing the muscle to relax. A build-up of acetylcholine in the synaptic cleft, due to the inhibition of cholinesterase, means the neurotransmitter continues to act on the muscle fibre, so that any nerve impulses are effectively continually transmitted.
Sarin acts on cholinesterase by forming a covalent bond with the particular serine residue at the active site. Fluoride is the leaving group, and the resulting phosphoester is robust and biologically inactive.[11][12]
Its mechanism of action resembles that of some commonly used insecticides, such as malathion. In terms of biological activity, it resembles carbamate insecticides, such as Sevin, and the medicines pyridostigmine, neostigmine, and physostigmine.
Degradation and shelf life

Rabbit used to check for leaks at sarin production plant, Rocky Mountain Arsenal (1970)
Sarin degrades after a period of several weeks to several months. The shelf life can be shortened by impurities in precursor materials. According to the CIA, some Iraqi sarin had a shelf life of only a few weeks, owing mostly to impure precursors.[16]
Its otherwise-short shelf life can be extended by increasing the purity of the precursor and intermediates and incorporating stabilizers such as tributylamine. In some formulations, tributylamine is replaced by diisopropylcarbodiimide (DIC), allowing sarin to be stored in aluminium casings. In binary chemical weapons, the two precursors are stored separately in the same shell and mixed to form the agent immediately before or when the shell is in flight. This approach has the dual benefit of solving the stability issue and increasing the safety of sarin munitions.
Effects and treatment
Sarin has a high volatility (ease with which a liquid can turn into a gas) relative to similar nerve agents, therefore inhalation can be very dangerous and even vapor concentrations may immediately penetrate the skin. A person’s clothing can release sarin for about 30 minutes after it has come in contact with sarin gas, which can lead to exposure of other people.[17] People who absorb a non-lethal dose but do not receive immediate appropriate medical treatment may suffer permanent neurological damage.Even at very low concentrations, sarin can be fatal. Death may follow in one minute after direct ingestion of a lethal dose unless antidotes, typically atropine and pralidoxime, are quickly administered.[4] Atropine, an antagonist to muscarinic acetylcholine receptors, is given to treat the physiological symptoms of poisoning. Since muscular response to acetylcholine is mediated through nicotinic acetylcholine receptors, atropine does not counteract the muscular symptoms. Pralidoxime can regenerate cholinesterases if administered within approximately five hours. Biperiden, a synthetic acetylcholine antagonist, has been suggested as an alternative to atropine due to its better blood–brain barrier penetration and higher efficacy.[18]
Sarin is 26 times more deadly than cyanide.[19] The LD50 of subcutaneously injected sarin in mice is 172 μg/kg.[20] Treatment measures have been described.[21]
Initial symptoms following exposure to sarin are a runny nose, tightness in the chest and constriction of the pupils. Soon after, the victim has difficulty breathing and experiences nausea and drooling. As the victim continues to lose control of bodily functions, the victim vomits, defecates and urinates. This phase is followed by twitching and jerking. Ultimately, the victim becomes comatose and suffocates in a series of convulsive spasms. Moreover, common mnemonics for the symptomatology of organophosphate poisoning, including sarin gas, are the "killer B's" of bronchorrhea and bronchospasm because they are the leading cause of death,[22] and SLUDGE - Salivation, Lacrimation, Urination, Defecation, Gastrointestinal distress, and Emesis.
Diagnostic tests
Controlled studies in healthy men have shown that a nontoxic 0.43 mg oral dose administered in several portions over a 3 day interval caused average maximum depressions of 22 and 30%, respectively, in plasma and erythrocyte cholinesterase levels. A single acute 0.5 mg dose caused mild symptoms of intoxication and an average reduction of 38% in both measures of cholinesterase activity. Sarin in blood is rapidly degraded either in vivo or in vitro. Its primary inactive metabolites have in vivo serum half-lives of approximately 24 hours. The serum level of unbound isopropylmethylphosphonic acid (IMPA), a sarin hydrolysis product, ranged from 2-135 µg/L in survivors of a terrorist attack during the first 4 hours post-exposure. Sarin or its metabolites may be determined in blood or urine by gas or liquid chromatography, while cholinesterase activity is usually measured by enzymatic methods.[23]History
Sarin was discovered in 1938 in Wuppertal-Elberfeld in Germany by scientists at IG Farben attempting to create stronger pesticides; it is the most toxic of the four G-Series nerve agents made by Germany. The compound, which followed the discovery of the nerve agent tabun, was named in honor of its discoverers: Schrader, Ambros, Gerhard Ritter and Van der Linde.[24]Use as a weapon
In mid-1939, the formula for the agent was passed to the chemical warfare section of the German Army Weapons Office, which ordered that it be brought into mass production for wartime use. A number of pilot plants were built, and a high-production facility was under construction (but was not finished) by the end of World War II. Estimates for total sarin production by Nazi Germany range from 500 kg to 10 tons.[25] Though sarin, tabun and soman were incorporated into artillery shells, Germany did not use nerve agents against Allied targets.- 1950s (early): NATO adopted sarin as a standard chemical weapon, and both the USSR and the United States produced sarin for military purposes.
- 1953: 20-year-old Ronald Maddison, a Royal Air Force engineer from Consett, County Durham, died in human testing of sarin at the Porton Down chemical warfare testing facility in Wiltshire, England. Ten days after his death an inquest was held in secret which returned a verdict of "misadventure". In 2004, the inquest was reopened and, after a 64-day inquest hearing, the jury ruled that Maddison had been unlawfully killed by the "application of a nerve agent in a non-therapeutic experiment."[26]
- 1956: Regular production of sarin ceased in the United States, though existing stocks of bulk sarin were re-distilled until 1970.
- March 1988: Over the span of two days in March, the ethnic Kurd city of Halabja in northern Iraq (population 70,000) was bombarded with chemical and cluster bombs, which included sarin, in the Halabja poison gas attack. An estimated 5,000 people died.[27]
- April 1988: Sarin was used four times against Iranian soldiers in April 1988 at the end of the Iran–Iraq War, helping Iraqi forces to retake control of the al-Faw Peninsula during the Second Battle of al-Faw. Using satellite imagery, the United States assisted Iraqi forces in locating the position of the Iranian troops during those attacks.[28]
- 1993: The United Nations Chemical Weapons Convention was signed by 162 member countries, banning the production and stockpiling of many chemical weapons, including sarin. It went into effect on 29 April 1997, and called for the complete destruction of all specified stockpiles of chemical weapons by April 2007.[29]
- 1994: The Japanese religious sect Aum Shinrikyo released an impure form of sarin in Matsumoto, Nagano, killing eight people and harming over 200. (see Matsumoto incident)
- 1995: Aum Shinrikyo sect released an impure form of sarin in the Tokyo Metro. Thirteen people died. (see Sarin gas attack on the Tokyo subway)
- 1998: In the US, Time Magazine and CNN ran false news stories alleging that in 1970 U.S. Air Force A-1E Skyraiders engaged in a covert operation called Operation Tailwind, in which they deliberately dropped sarin-containing weapons on U.S. troops who had defected in Laos. CNN and Time Magazine later retracted the stories and fired the producers responsible.[30]
- 2004: Iraqi insurgents detonated a 155 mm shell containing binary precursors for sarin near a U.S. convoy in Iraq. The shell was designed to mix the chemicals as it spins during flight. The detonated shell released only a small amount of sarin gas, either because the explosion failed to mix the binary agents properly or because the chemicals inside the shell had degraded with age. Two United States soldiers were treated after displaying the early symptoms of exposure to sarin.[31]
- 21 August 2013: Sarin was used in an attack in the Ghouta region of the Rif Dimashq Governorate of Syria during the Syrian civil war.[32] Varying[33] sources gave a death toll of 322[34] to 1,729, and said that none of the victims had physical wounds.[35]


No comments:
Post a Comment